clinical trial phases – clinical trial phases explained
Clinical trials follow a typical series from early, small-scale, Phase 1 studies to late-stage, large scale, Phase 3 studies, What are the Clinical Trial Phases? Watch this video to learn about
The timeline for clinical trial phases might look like this:, Basic Research/Drug Development and Pre-Clinical/Translational Research combined 3 to 6 years: Phase 1, Phase 2, and Phase 3 Clinical Trials combined 6 to 7 years: FDA Review/Manufacturing: 0,5 to 2 years: Phase 4 Clinical Trial/Post-Market Surveillance/Report Adverse Events
Temps de Lecture Estimé: 7 mins
· Clinical trials are generally classified into Phases I to IV, It is not possible to draw distinct lines between the phases, and diverging opinions about definitions and methodology do exist, An investigational product can be evaluated in more than one phase simultaneously in different clinical trials, and some clinical trials may overlap two different phases,
Clinical Trial Phases
Phases of Clinical Trials
· Fichier PDF
Clinical trials in human medicines
· Clinical trials are run in multiple steps called phases that build on one another Each phase helps answer different questions about the new treatment While the treatment’s safety and efficacy is monitored throughout each phase, the phase that a clinical trial is in roughly represents how much is known about the treatment that’s being studied,
Phases of clinical research
Phases of Clinical Trials Preclinical Trial Usually done on animals to determine the drug is safe enough for human testing Phase I Determine Pharmacological actions and Tolerability* Phase II Evaluate Safety and Efficacy Phase III Evaluate Effectiveness** and risk-benefit ratio Phase IV Monitor long term effects and effectiveness Duration 3-6 years since the drug discovery Months
Clinical Trial Phases: What Happens in Phase 0 I II III
Phases of Clinical Trials
Phases of clinical trials
Key points of phase I clinical trials The first few people in the study get a very low dose of the treatment and are watched very closely, If there are only Phase I trials are also looking at what the drug does to the body and what the body does with the drug, Safety is the main concern, The
Phase 0 Clinical Trials: Exploring If and How A New Drug May WorkEven though phase 0 studies are done in humans, this type of study isn’t like the other phases of clinical trials, The purpose of this phase is toPhase I Clinical Trials: Is The Treatment Safe?Phase I studies of a new drug are usually the first that involve people, The main reason for doing phase I studies is to find the highest dose of tPhase II Clinical Trials: Does The Treatment Work?If a new treatment is found to be reasonably safe in phase I clinical trials, it can then be tested in a phase II clinical trial to find out if itPhase III Clinical Trials: Is It Better Than What’S Already available?Treatments that have been shown to work in phase II studies usually must succeed in one more phase of testing before they’re approved for general uSubmission For FDA Approval: New Drug Application NDAIn the United States, when phase III clinical trials or sometimes phase II studies show a new drug is more effective and/or safer than the currenPhase IV Clinical Trials: What Else Do We Need to Know?Drugs approved by the FDA are often watched over a long period of time in phase IV studies, Even after testing a new medicine on thousands of peopl
Users are able to view: the description of phase-II to phase-IV adult clinical trials where the investigator sites are in the EEA; the description of any clinical trials in children with investigator sites in the EU and any trials that form part of a PIP including those where the investigator sites are outside the EU,
Types and Phases of Clinical Trials
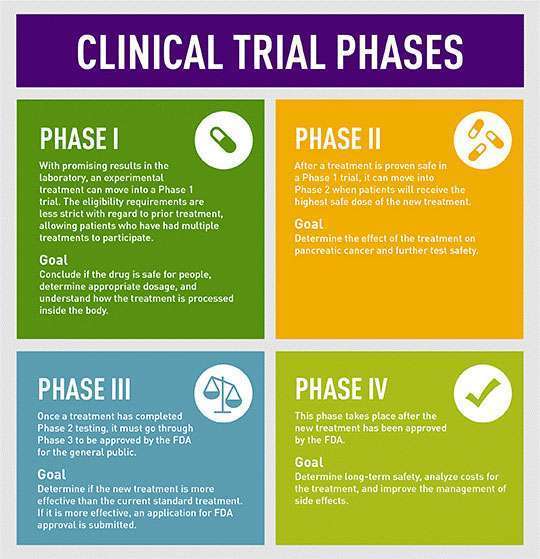
Phases of clinical trials
What Are Trial phases?
Clinical Trials Phases: Definition of Phase 1 2 3 & 4
the four phases of clinical trials The process of learning about and developing an investigational medicine is divided into four phases At first, very few people receive the medicine being studied,
Step 3: Clinical Research
Ultimate Guide to Clinical Trial Phases
Clinical trials of biomedical interventions typically proceed through four phases, Phase I Clinical Trial, Phase I clinical trials are done to test a new biomedical intervention for the first time in a small group of people e,g, 20-80 to evaluate safety e,g, to determine a safe dosage range and identify side effects, Phase II Clinical Trial
clinical trial phases
Clinical trials testing potential medical products are commonly classified into four phases, The drug development process will normally proceed through all four phases over many years, [1] If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population, [1]
· In this guide, you will learn what clinical trials are, what types exist, and the details regarding the five different phases: phase 0, phase I, phase II, phase III, and phase IV, We address your frequently asked questions and explore related topics, and also include a clinical trial phase chart and a phase …
Clinical Trial Phases 1 2 3 & 4: Find FDA Clinical Trial
· Phase III of a clinical trial usually involves up to 3,000 participants who have the condition that the new medication is meant to treat, Trials in this phase can last for several years,