ev 201 study – enfortumab vedotin nejm
EV-201 Cohort 2 Study: 89 patients previously treated with a PD-1/L1 inhibitor and not eligible for platinum-based chemotherapy Serious adverse reactions occurred in 39% of patients treated with PADCEV; the most common ≥3% were pneumonia sepsis and diarrhea 5% each Fatal adverse reactions occurred in 8% of patients including acute kidney injury 22%, metabolic acidosis, sepsis
EV-201 study: A single-arm open-label multicenter study of enfortumab vedotin for treatment of patients with locally advanced or metastatic urothelial cancer who previously received immune checkpoint inhibitor therapy Jonathan E Rosenberg, x, Jonathan E, Rosenberg, Search for articles by this author, Elisabeth I, Heath, x, Elisabeth I, Heath, Search for articles by this author, Peter H, O
Cited by : 9
EV-201: Results of enfortumab vedotin monotherapy for
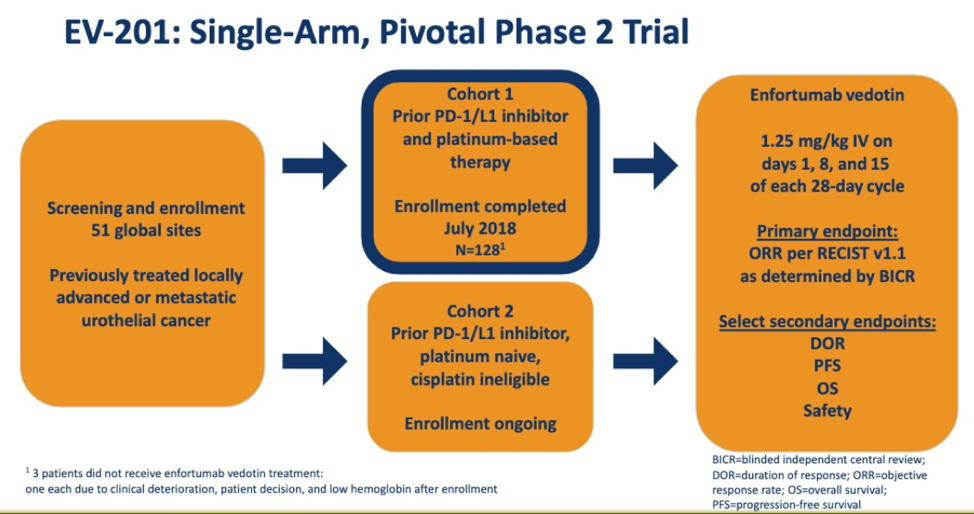
· EV-201 is an open-label multicenter multinational study in which patients received 125 mg/kg EV on Days 1 8 and 15 of each 28-day cycle The EV-201 trial design is as follows: The EV-201 …
EV-201 study: A single-arm open-label multicenter study
EV-201 Cohort 2 Study: 89 patients previously treated with a PD-1/L1 inhibitor and not eligible for platinum-based chemotherapy Serious adverse reactions occurred in 39% of patients treated with PADCEV; the most common ≥3% were pneumonia sepsis and diarrhea 5% each Fatal adverse reactions occurred in 8% of patients including acute kidney injury 2,2%, metabolic acidosis, sepsis
· A Study of Enfortumab Vedotin for Patients With Locally Advanced or Metastatic Urothelial Bladder Cancer EV-201 The safety and scientific validity of this study is the responsibility of the study …
Actual Enrollment : 219 participants
EV-201 study: A single-arm open-label multicenter study
EV-201 is a pivotal single-arm two-cohort study of EV in la/mUC patients with prior CPI and platinum-containing chemotherapy Cohort 1 or a CPI and no prior chemotherapy Cohort 2 Here we present preliminary data from Cohort 1 Methods: Pts in this open-label multicenter study received 1,25 mg/kg EV on Days 1, 8, and 15 of each 28-day
Cited by : 26
Pivotal Trial of Enfortumab Vedotin in Urothelial
The EV-201 study will assess the antitumor activity and safety of enfortumab vedotin to support potential registration under the U,S, Food and Drug Administration’s FDA accelerated approval regulations, The primary endpoint of the single-arm, open-label trial is confirmed objective response rate ORR, per independent review, Secondary endpoints include assessments of overall survival
Enfortumab vedotin after PD-1 or PD-L1 inhibitors in
A Study of Enfortumab Vedotin for Patients With Locally
In the phase I EV-101 study of enfortumab vedotin 112 patients received EV the majority of whom received prior platinum chemotherapy 81% and a checkpoint inhibitor 75% and this patient population had an objective response rate of 33% The median overall survival OS was reported to be 12,5 months 2 In this study the authors describe the results of EV-201 EV monotherapy for locally
A Study of Enfortumab Vedotin Alone or With Other
· 1573 – EV-201: A single-arm open-label multicenter study of enfortumab vedotin for treatment of patients with locally advanced or metastatic urothelial cancer who previously received immune checkpoint inhibitor therapy Date 22 Oct 2018 Session Poster display session: Breast cancer – early stage, locally advanced & metastatic, CNS tumours, Developmental therapeutics, Genitourinary tumours
ASCO 2019: EV-201: Results of Enfortumab Vedotin
· EV-201 is a multicentre single-arm phase 2 study of enfortumab vedotin in patients with locally advanced or metastatic urothelial carcinoma previously treated with PD-1 or PD-L1 inhibitors Cohort 2 included adults aged ≥18 years with an Eastern Cooperative Oncology Group performance status score of 2 or less who were considered ineligible for cisplatin at enrolment and who had not
EV-201: A single-arm open-label multicenter study of
· This study will examine the safety and anticancer activity of enfortumab vedotin EV given intravenously as monotherapy and in combination with other anticancer therapies as first line 1L and second line 2L treatment for patients with urothelial cancer The primary goal of the study is to determine the safety tolerability and efficacy of enfortumab vedotin alone and in combination with
ASCO GU 2021: Enfortumab Vedotin In Cisplatin-Ineligible
Methods: EV-201 is a global phase II single-arm study of enfortumab vedotin 125 mg/kg intravenously on days 1 8 and 15 of every 28-day cycle in patients with locally advanced or metastatic urothelial carcinoma who were previously treated with platinum chemotherapy and anti-PD-1/L1 therapy The primary end point was objective response rate per Response Evaluation Criteria in Solid Tumors
PADCEV Study Overview: Post–PD-L1 Cisplatin-Ineligible
NCT03219333 Clinical Trial
EV-201 study: A single-arm open-label, multicenter study of enfortumab vedotin for treatment of patients with locally advanced or metastatic urothelial cancer who previously received immune
PADCEV Study Overview: Patients With la/mUC Post-Platinum
ev 201 study