nh3 molar mass – calculate molar mass of nh3
Masse molaire of NH3 amoniaco
Ammonia
Composition of Ammonia – NH 3, Element, Symbol, Atomic Mass, # of Atoms, Mass Percent, Nitrogen, N, 14,0067 g/mol,
Molar mass of NH3 is 17,03052 ± 0,00041 g/mol Compound name is ammonia Convert between NH3 weight and moles
Molar mass of ammonia is 17,03052 ± 0,00041 g/mol Compound name is ammonia Convert between NH3 weight and moles
· The molar mass of NH3 ammonia is 17,031 g per mole, The chemical properties of ammonia include ammonia having high stability, being combustible in air, and forming nitric oxide when combined with a platinum-rhodium catalyst at approximately 800°C, What’s A Mole? The molar mass of a chemical substance is the mass which is possessed by a single mole of that substance, In order to understand
Molar mass: 17,031 g/mol Appearance Colourless gas Odor: strong pungent odour Density: 0,86 kg/m 3 1,013 bar at boiling point 0,769 kg/m 3 STP 0,73 kg/m 3 1,013 bar at 15 °C 681,9 kg/m 3 at −33,3 °C liquid See also Ammonia data page 817 kg/m 3 at −80 °C transparent solid Melting point
Molar mass of *4NH3
Molar Mass, Molecular Weight and Elemental Composition Calculator Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of *4NH3 is 68,1221 g/mol
Massa molar of NH3
Masse molaire of NH3amoniaco
Molecular weight of NH3-N
· Explanation of how to find the molar mass of NH3: Ammonia,A few things to consider when finding the molar mass for NH3:- make sure you have the correct chemi
Auteur : Wayne Breslyn
What is the molar mass of ammonia, “NH”_3?
Massa molar of NH3 is 17,03052 ± 0,00041 g/mol Convert between NH3 weight and moles
Molar mass of NH3
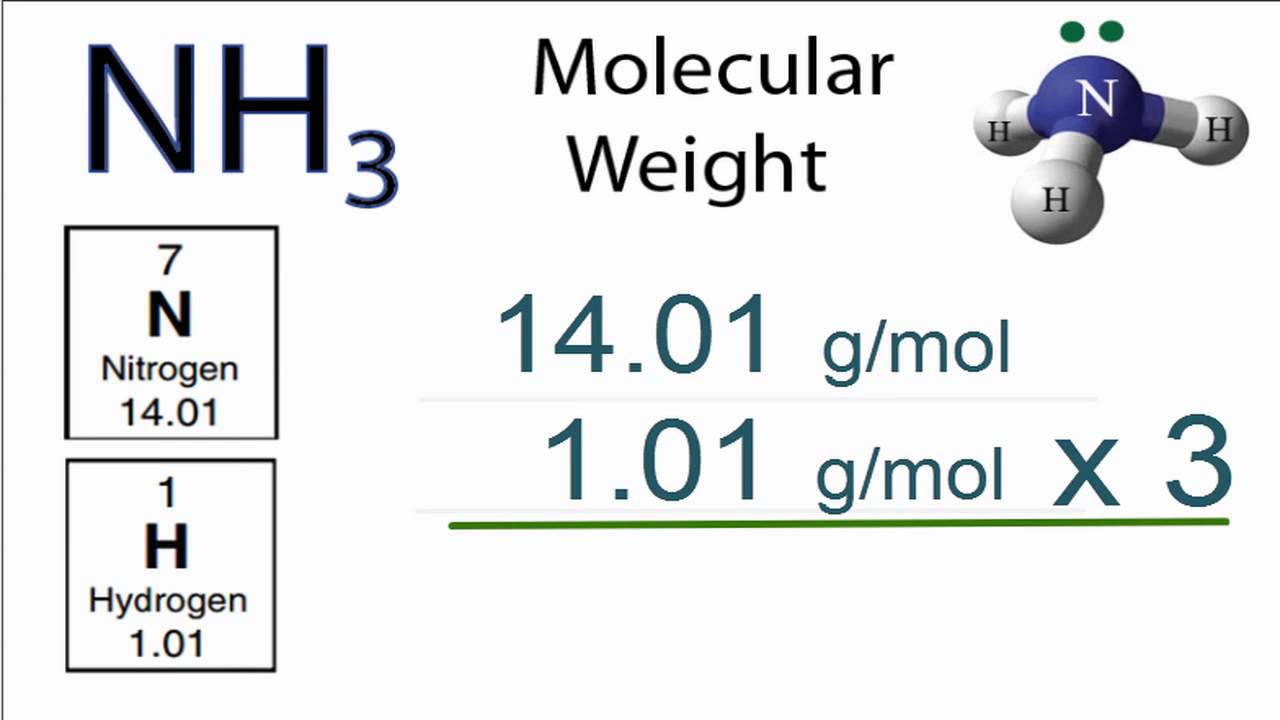
· To determine the molar mass of any compound, all you have to do is add up the molar masses of every atom that makes up the respective compound,, In this case, you know that ammonia, #”NH”_3#, is composed of , one nitrogen atom, #”N”#; three hydrogen atoms, #”H”#; This means that its molar mass will be the sum of the molar mass of one nitrogen atom and three times the molar mass of a hydrogen atom,
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together, Finding molar mass starts with units of grams per mole g/mol, When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance, The formula weight is simply the weight in atomic mass
Ammonia NH3 Molar Mass
NH3 Ammonia Molar Mass And Chemical Properties
The molar mass and molecular weight of NH 3 is 17,0305,
Molecular weight of NH3
NH3 Molar Mass
nh3 molar mass
›› NH3 molecular weight, Molar mass of NH3 = 17,03052 g/mol, This compound is also known as Ammonia, Convert grams NH3 to moles or moles NH3 to grams, Molecular weight calculation: 14,0067 + 1,00794*3 ›› Percent composition by element
Molar mass of ammonia
Molar Mass / Molecular Weight of NH3 Ammonia