udi medical devices fda – unique device identification
UDI Rule and Guidances, Training, Resources, and Dockets
· Include a unique device identifier UDI, issued under an FDA-accredited issuing agency’s UDI system, on device labels, device packages, and in some instances, directly on the device,
Explorez davantage
| Unique Device Identification UDI – Healthcare , GS1 | www,gs1,org |
| UDI is new with the Medical Device Regulation | easymedicaldevice,com |
| AccessGUDID – Identify Your Medical Device | accessgudid,nlm,nih,gov |
| Unique Device Identification UDI System | ec,europa,eu |
| 21 CFR § 801,20 – Label to bear a unique device | www,law,cornell,edu |
Recommandé pour vous en fonction de ce qui est populaire • Avis
Compliance Dates for UDI Requirements
Unique Device Identification UDI – Healthcare
Unique Device Identification System UDI System
Global Unique Device Identification Database GUDID
The labels and packages of class II medical devices must bear a UDI, § 801,20, Dates on the labels of these devices must be formatted as required by § 801,18,
udi medical devices fda
Unique Device Identification UDI requirements for medical devices have been implemented by the US Food and Drug Administration FDA The FDA issued the final Unique Device Identifier UDI Rule and published it in the US Federal Register on 24 September 2013, The UDI system final rule provides standard device identification and accompanying
Developing a UDI Using an FDA-Accredited Issuing Agency’s System, To develop a UDI, device labelers must contact one of the issuing agencies accredited by the FDA, See Contact an FDA-Accredited
CFR
Medical Devices
· On the date a medical device must bear a UDI on its label, any NHRIC or NDC numbers assigned to the device are rescinded and may no longer be on the device label or package 21 CFR 801,57a, If
Temps de Lecture Estimé: 12 mins
FDA UDI Help Desk
Unique Device Identifier – UDI
· The following types of devices are excepted from the requirement of § 801,20; a device within one or more of the following exceptions is not required to bear a unique device identifier UDI: 1 A finished device manufactured and labeled prior to the compliance date established by FDA for § 801,20 regarding the device, This exception expires with regard to a particular device 3 years after the compliance date established by FDA for the device,
Medical Device Accessories
Medical Devices; Device Advice: Comprehensive Regulatory Assistance; Unique Device Identification System UDI System FDA UDI Help Desk; Unique Device Identification System UDI System
UDI Basics
The US Food and Drug Administration FDA released in September 2013 a UDI rule which establishes a UDI system applying to all medical devices placed on the US market, On 17 December 2013, GS1 has been accredited by the US FDA as issuing agency for unique device identifiers UDIs,
Medical Device UDI Unique Device Identification
The Global Unique Device Identification Database GUDID is a database administered by the FDA that will serve as a reference catalog for every device with a unique device identifier UDI, GUDID
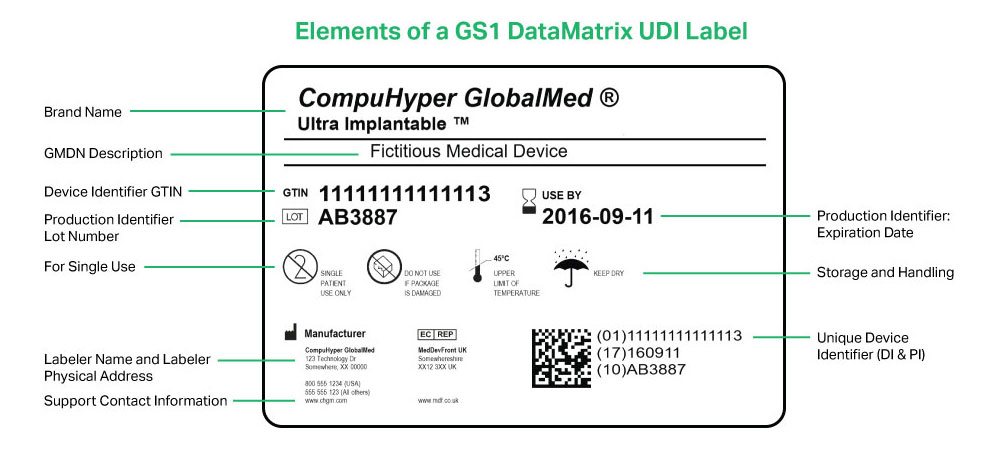
· The unique device identification UDI is a unique numeric or alphanumeric code related to a medical device, It allows for a clear and unambiguous identification of specific devices on the market and facilitates their traceability, The UDI comprises the following components, These provide access to useful information about the device,
| Unique Device Identification UDI System | 26/05/2021 |
| Overview , Public Health – European Commission | 11/12/2020 |
| EOValue | 04/08/2019 |
| Call for applications in the view of the designation of |
Afficher plus de résultats
· The UDI requirements apply to all medical devices per 21 CFR 801,20, including medical device accessories, unless an exception or alternative applies or was granted pursuant to 21 CFR 801,30 or
· UDI Form and Content: Unique Device Identification System: Form and Content of the Unique Device Identifier UDI – Guidance for Industry and Food and Drug Administration Staff: 07/01/2020
UDI Exceptions, Alternatives, and Time Extensions
FDA regulates the sale of medical device products in the U,S, and monitors the safety of all regulated medical products,